Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the terms atom, ion, molecule, molecular ion, and free radical.
ii. Differentiate between atoms, ions, molecules, molecular ions, and free radicals based on their characteristics and formation.
iii. Recognize and classify various chemical species as atoms, ions, molecules, molecular ions, or free radicals.
Introduction
The realm of chemistry is filled with a captivating array of particles, each playing a distinct role in shaping the matter that surrounds us. In this lesson, we embark on an exploration of five fundamental types of chemical species: atoms, ions, molecules, molecular ions, and free radicals.
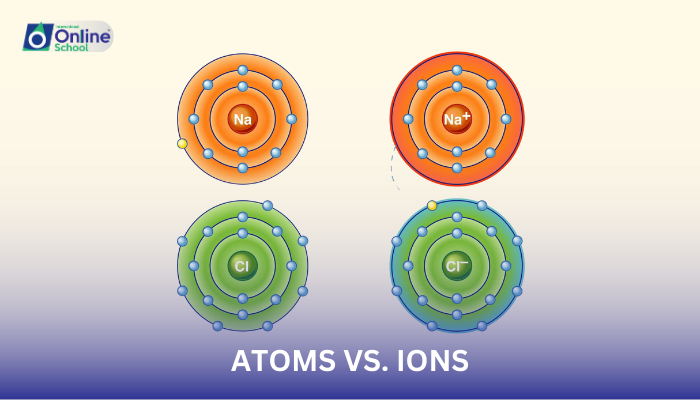
i. Atoms: The Building Blocks of Matter
Atoms, the smallest units of matter that can participate in chemical reactions, are the fundamental building blocks of all matter. They consist of a positively charged nucleus, surrounded by a cloud of negatively charged electrons. Atoms are classified based on their atomic number, which represents the number of protons in their nucleus.
Examples of atoms include:
Hydrogen (H)
Oxygen (O)
Carbon (C)
Gold (Au)
Silver (Ag)
ii. Ions: Charged Particles
Ions are atoms or groups of atoms that have gained or lost electrons, resulting in a net positive or negative charge. Cations are positively charged ions, while anions are negatively charged ions. Ions are formed when atoms gain or lose electrons to achieve a stable electron configuration.
Examples of ions include:
Sodium ion (Na+)
Chloride ion (Cl-)
Magnesium ion (Mg2+)
Sulfate ion (SO42-)
iii. Molecules: A Union of Atoms
Molecules are groups of two or more atoms that are held together by chemical bonds. These bonds can be formed through the sharing or transfer of electrons between atoms. Molecules can be either neutral, having no net charge, or charged, known as molecular ions.
Examples of molecules include:
Water (H2O)
Carbon dioxide (CO2)
Sugar (C6H12O6)
iv. Molecular Ions: Charged Molecular Species
Molecular ions are polyatomic ions that have a net charge. They are formed when a group of atoms loses or gains electrons, resulting in a charged molecule. Molecular ions play a crucial role in various chemical reactions and processes.
Examples of molecular ions include:
Ammonium ion (NH4+)
Hydroxide ion (OH-)
Cyanide ion (CN-)
v. Free Radicals: Highly Reactive Particles
Free radicals are atoms or molecules with unpaired electrons, making them highly reactive. These unpaired electrons create a magnetic field and a tendency to react with other molecules to form new compounds. Free radicals play a significant role in various biological processes, including energy production and cellular signaling.
Examples of free radicals include:
Hydrogen radical (H•)
Oxygen radical (O•)
Hydroxyl radical (OH•)
vi. Distinguishing Atoms, Ions, Molecules, Molecular Ions, and Free Radicals
The key to distinguishing among atoms, ions, molecules, molecular ions, and free radicals lies in their characteristics and formation:
Atoms: The smallest units of matter, not bound to other atoms.
Ions: Charged particles formed by gaining or losing electrons.
Molecules: Groups of atoms held together by chemical bonds.
Molecular Ions: Charged molecules with a net charge.
Free Radicals: Atoms or molecules with unpaired electrons.
The world of atoms, ions, molecules, molecular ions, and free radicals offers a fascinating glimpse into the fundamental building blocks of matter and the intricate processes that govern chemical interactions. Understanding these diverse species is essential for comprehending the intricate tapestry of chemistry and its influence on the world around us.